Rydberg Constant: Experimental Determination Using Atomic Spectra
The Rydberg constant is a fundamental physical constant that plays a vital role in understanding the atomic spectra of elements, particularly hydrogen. It forms the backbone of the Rydberg formula, which helps in predicting the wavelengths of light emitted by atoms when electrons transition between energy levels.
In this post, we’ll break down how the Rydberg constant is experimentally determined using spectroscopic techniques.
🔬 What is the Rydberg Constant?
The Rydberg constant (R∞) is used in the Rydberg formula to describe the wavelengths of light emitted when electrons in an atom drop from a higher energy level to a lower one.
Rydberg Formula:
[latex]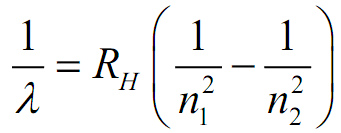
Where:
- λ = Wavelength of emitted light
- n₁ and n₂ = Energy level integers (n₂ > n₁)
- R∞ = Rydberg constant
🌈 Atomic Spectra and Electron Transitions
Atoms, when excited, have electrons that jump to higher energy levels. As they return to their original or lower levels, they emit light at specific wavelengths, creating distinct spectral lines. These patterns are unique to each element and are called atomic spectra.
This phenomenon forms the foundation of spectroscopy, the study of the interaction between matter and electromagnetic radiation.
🔍 How is the Rydberg Constant Determined Experimentally?
To determine the Rydberg constant, scientists measure the precise wavelengths of light emitted during electron transitions and apply the Rydberg formula.
Key Experimental Techniques:
- Hydrogen Spectrum
- Hydrogen is ideal due to its simple atomic structure and clearly defined spectral lines.
- Spectrometers
- Instruments that split light into its component wavelengths, allowing for accurate measurement of spectral lines.
- Diffraction Gratings
- Devices inside spectrometers that use interference to separate light based on wavelength.
- Digital Cameras
- In cost-effective setups, digital cameras capture the emitted spectra, which are then analyzed using software tools.
🧪 Typical Experimental Values of the Rydberg Constant
The accepted value of the Rydberg constant is:
R∞ ≈ 10,973,731.56816 m⁻¹
Values determined using different spectroscopic methods closely match this, proving the precision and reliability of experimental techniques.
✅ Summary
Spectroscopic analysis of the hydrogen atomic spectrum provides a reliable way to experimentally calculate the Rydberg constant. Using tools like spectrometers, diffraction gratings, and modern digital imaging, scientists can accurately measure spectral lines and apply the Rydberg formula to determine this key physical constant.
📌 FAQs on Rydberg Constant
Q1: Why is hydrogen commonly used to determine the Rydberg constant?
A: Hydrogen has a simple atomic structure with clearly defined spectral lines, making it ideal for accurate measurements.
Q2: What is the value of the Rydberg constant?
A: The accepted value is approximately 10,973,731.56816 m⁻¹.
Q3: Can digital cameras be used in spectroscopy?
A: Yes, in low-cost setups, digital cameras can capture emission spectra for analysis.
Q4: What role does the Rydberg formula play in atomic physics?
A: It helps predict the wavelengths of light emitted by atoms during electron transitions.

